Detect-ME: EndoSign Cell Collection Device Study
Esophageal cancer is the sixth leading cause of cancer-related deaths worldwide1. Your participation in this study will help make important discoveries related to GERD, Barrett’s esophagus and esophageal cancer screening. Identifying patients at the precancerous stage, gives the ability to intervene early with curative therapy options.
Study Snapshot
Detect-ME: Detection and Monitoring of Barett's Esophagus and Esophageal Neoplasia Using Methylation Biomarkers on Endosign Cell Collection Device
Purpose:
The goal of this study is to help develop a convenient diagnostic test that can be conducted in a clinic to detect and monitor conditions such as GERD and Barrett’s esophagus to prevent esophageal cancer. The study is sponsored by Cyted Health.
Eligibility:
Individuals with known Barrett's esophagus (a documented history of >1 cm BE), with a surveillance endoscopy scheduled.
or
Individuals with chronic reflux (GERD) scheduled for a high-risk screening (initial endoscopy) looking for Barrett's esophagus and at least 3 or more additional risk factors including:
- Age ≥ 50
- Family history of Barrett’s or esophageal cancer
- Current or Former Smoker
- Obesity
- Male
- Non-Hispanic White
Both individuals must be willing to undergo non-endoscopic sampling with the study device prior to EGD (up to 6 weeks prior to EGD or day of EGD).
Participation:
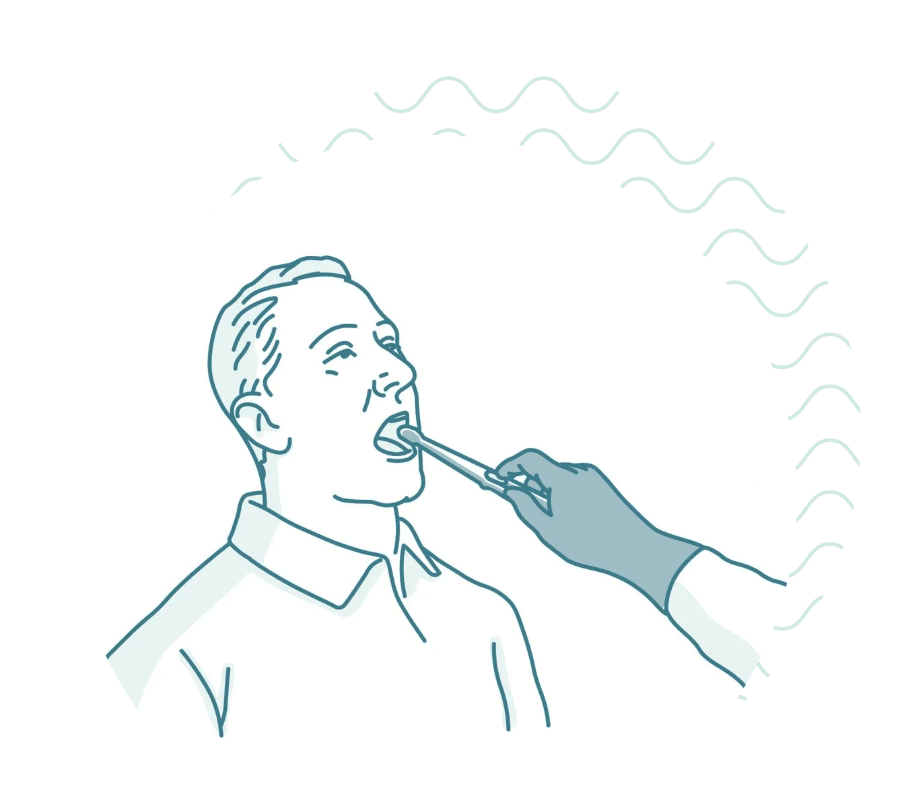
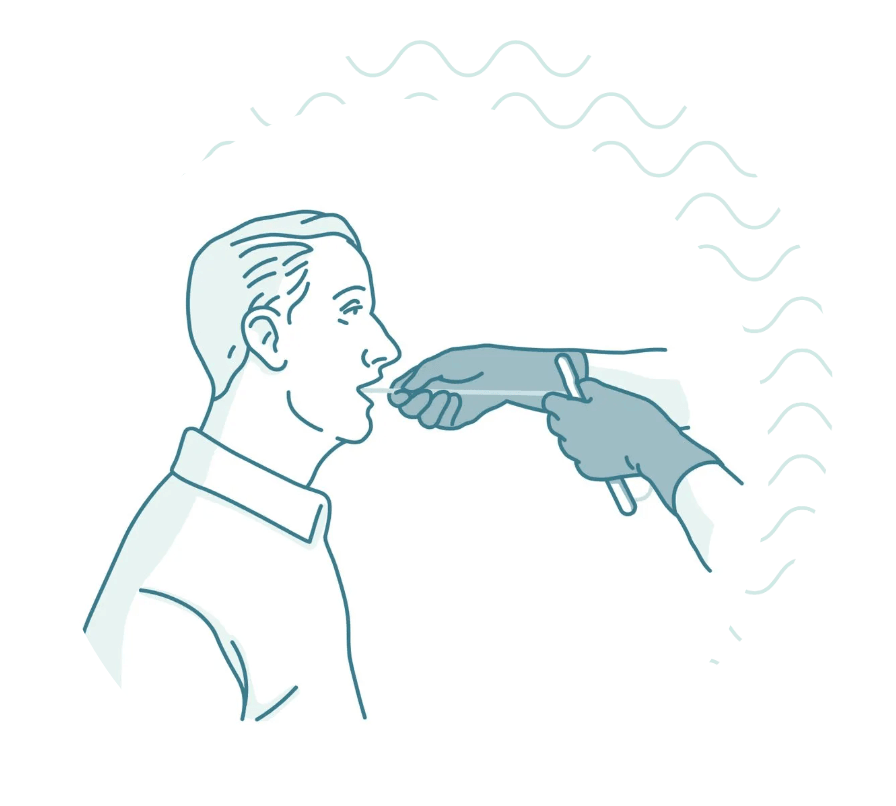
Participation would consist of coming in to a nearby research clinic where you would swallow a small capsule similar in size to a vitamin pill. The capsule contains a tiny sponge attached to a surgical thread, which the clinician would then gently retrieve collecting cells from the entire length of your esophagus. The actual procedure can be completed in less than 20 minutes and there is no need for sedation.
Compensation:
Participants will receive $100 for their time after completing their participation in the study.
Participating is simple
1Chat with a Study Coordinator
A brief phone conversation with one of our study coordinators will quickly determine if you meet the eligibility criteria for the study.
2Review and Sign an Informed Consent Form
If you meet the eligibility requirements, the coordinator will guide you through the Informed Consent answering any questions you may have. The clnic team will then contact you to schedule your appointment at a convenient location.
3Take the Test at a Nearby Clinic
You will be asked to swallow a small capsule similar in size to a vitamin pill with a cup of water. The capsule contains a tiny sponge attached to a surgical thread. After a few minutes, the clinician will then gently retrieve it. After a brief survey, you're done.
4Attend Your Endoscopy, as Scheduled
Simply attend your appointment with your physician as scheduled.
We are committed to empowering patients and clinicians to participate in making clinical research more accessible, inclusive and impactful.

What To Expect
Participating is simple, painless and quick.

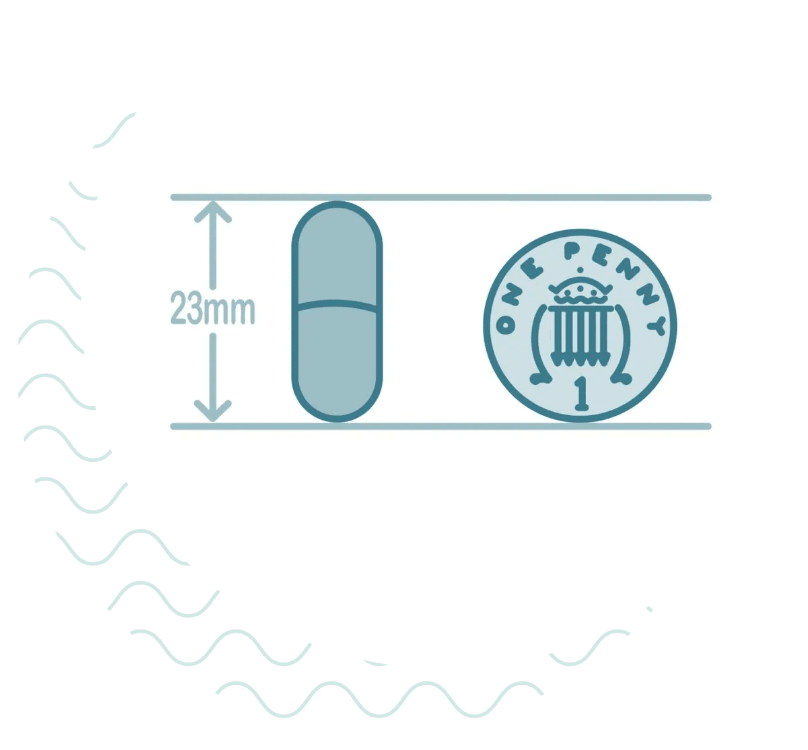
How big is the capsule?
The capsule is about the size of a vitamin pill and contains a sponge connected to a thin, strong surgical thread.

How does the testing work?
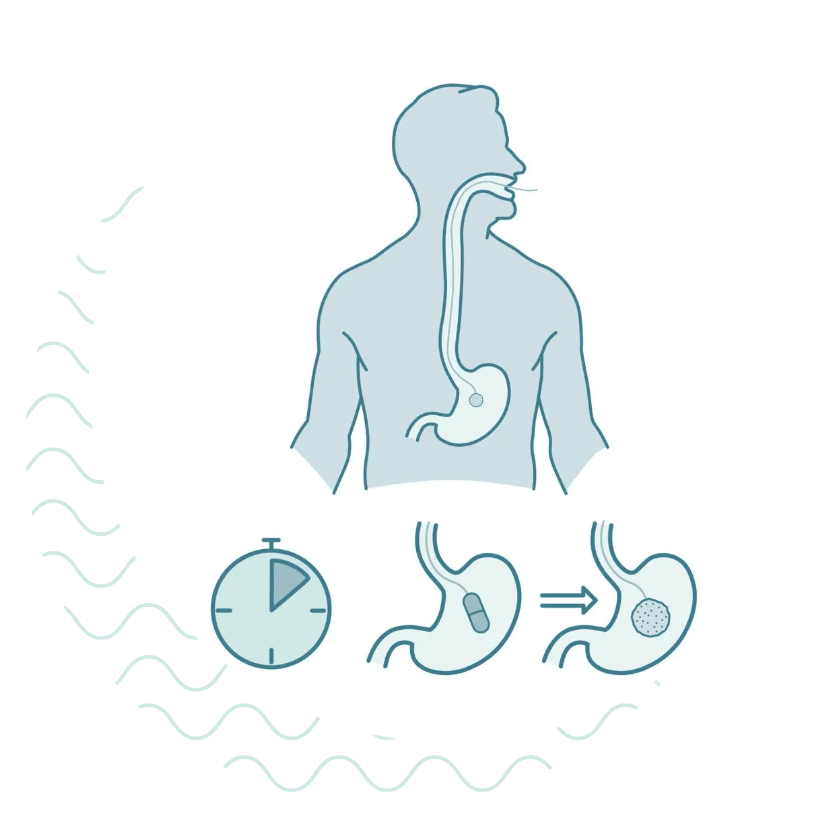
1. You will be asked to swallow the capsule with 1 or 2 cups of water.

2. The capsule dissolves when it reaches your stomach, releasing the sponge. This takes 7 minutes.

3. The clinician will then gently pull the thread, and the sponge will collect cells as it passes through your esophagus. This part only takes a few seconds. Because it collects cells from the entire length of the esophagus, it covers a larger area than a traditional biopsy.

Are there any risks?
There have been no serious side effects reported and in over 7,500 uses, there have been zero instances of the sponge detaching from the string. Most people have no issues swallowing the capsule, but the test will be stopped if you experience any problems or discomfort. Some people may have a mild sore throat after the test, but this usually goes away within 48 hours.

Is it uncomfortable?
Most people have no issues swallowing the capsule, but the test will be stopped if you experience any problems or discomfort. Some people report gagging as the sponge is retrieved and may have a mild sore throat after the test, but this usually goes away within 48 hours.
Let's Talk
Just a small amount of your time could make a big difference.
Click the link below to connect with a study coordinator and find out if you're eligible to participate.

AH-DETECT-004-5-25
Copyright 2025, Aton Holdings, Inc.